 The Kenya Pharmacy and Poisons Board has issued a warning about fake Ozempic Pens circulating in the market following an alert from Interpol. The board explained in a statement that it received an alert from Interpol concerning pens used to treat type 1 and 2 diabetes that had been falsely labeled.
The Kenya Pharmacy and Poisons Board has issued a warning about fake Ozempic Pens circulating in the market following an alert from Interpol. The board explained in a statement that it received an alert from Interpol concerning pens used to treat type 1 and 2 diabetes that had been falsely labeled.
Additionally, the board indicated that Ozempic Pens are not registered or authorized for sale in the Kenyan market. Therefore, any products marketed as Ozempic Pens are illegal.
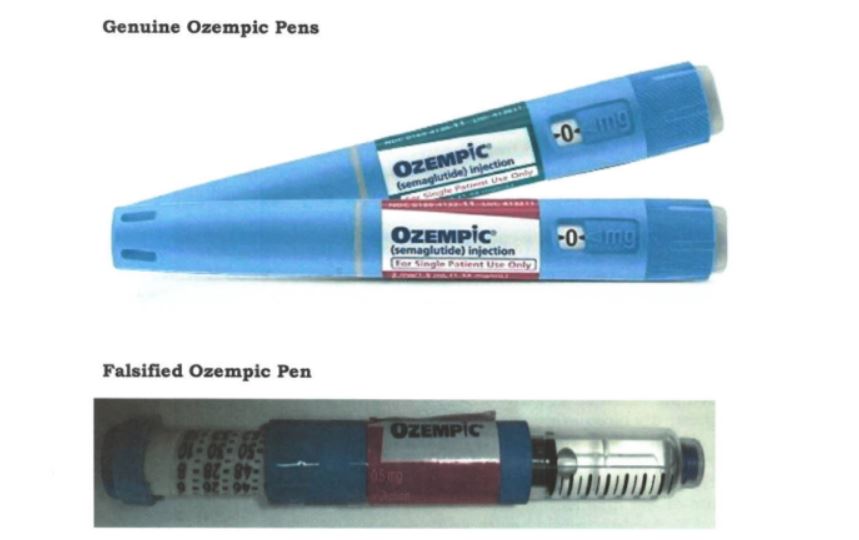
“An alert from Interpol concerns the falsification of Ozempic Pens (Semaglutide), where Apidra Solostar pens (glulisine) used to treat both type 1 and type 2 diabetes have been falsely relabeled as Ozempic (Semaglutide) Pens. Any product marketed as Ozempic Pens is illegal, and the Board cannot ascertain their safety, quality, and effectiveness,” the statement read in part.
To ensure the falsified pens are not circulating in the market, the board has initiated a rapid response initiative.
“To safeguard public safety, the Board has initiated a rapid response and heightened surveillance to verify whether the falsified Ozempic (Semaglutide) Pens are presently circulating in the Kenyan market,” the statement read.
Additionally, Kenyans have been cautioned against purchasing, distributing, or retailing the products. Medical practitioners have also been warned against administering the pen to patients.
“The Board cautions the public and healthcare professionals against trading, distributing, wholesaling, retailing, issuing, dispensing, using, or administering the falsified Ozempic (Semaglutide) Pens, as such actions are illegal and jeopardize public health and safety.
“We encourage the public and healthcare professionals to immediately share any information regarding Ozempic Pens with the Pharmacy and Poisons Board,” the board urged.








