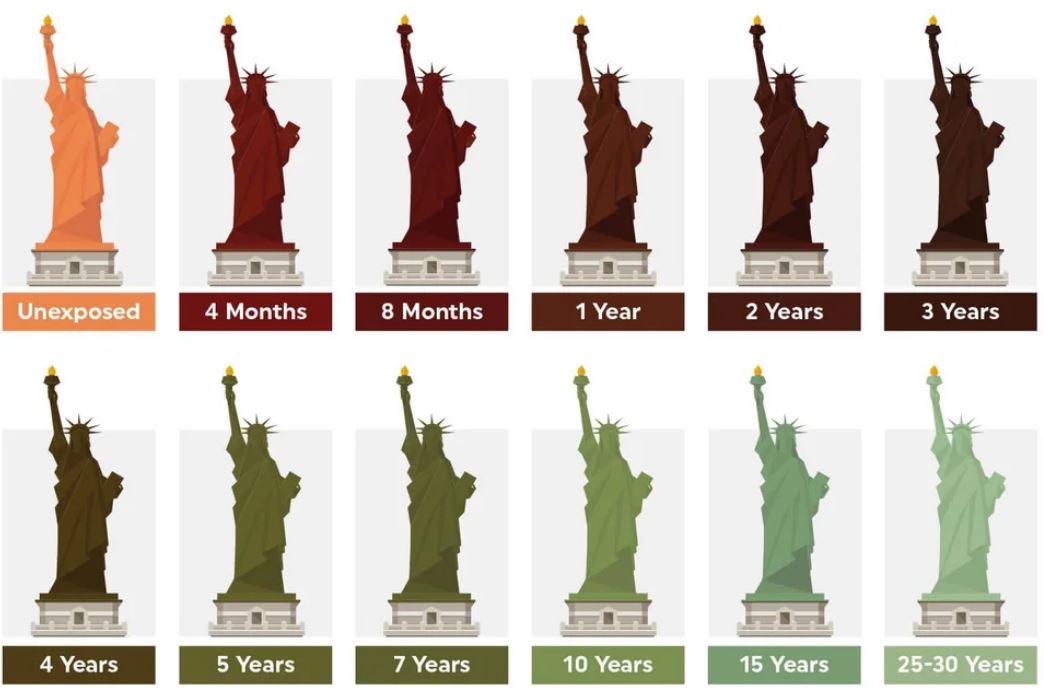
Many people are surprised to learn that the Statue of Liberty was not always blue-green in color.
The iconic color that it has come to be associated with is as a result of natural processes. When it was unveiled in 1886, the Statue of Liberty was a shiny brown color. That’s the same color as a penny, because they are both covered with copper.
Over the years, the copper reacted with oxygen to form copper carbonates, copper sulfide, and copper sulfate.
The patina that is formed through this reactions actually protects the underlying metal from corrosion and degradation. That explains the longevity of the Statue.
Here’s a simple graphic explaining how that process works.